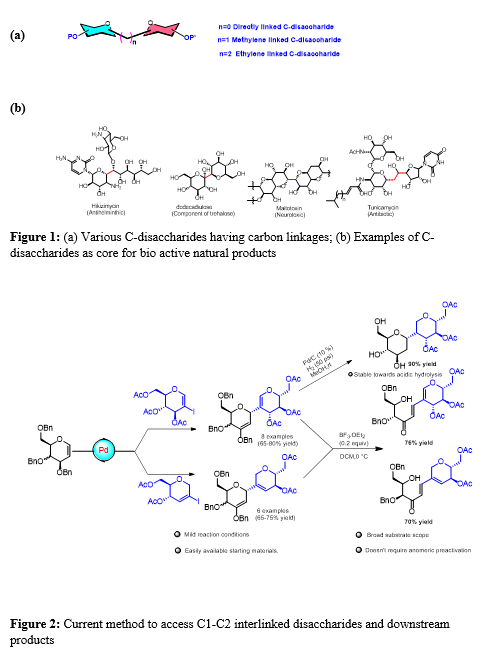
C-disaccharides are enzymatically stable analogues of O-glycosides. They are interconnected by carbon spacer (Fig 1a). Above class of molecules find application as therapeutics in different diseases areas and constitute core structure of several bioactive natural products (Fig. 1b). However, stereo-selective synthesis of such linkages remains a challenge.
A novel and convenient method for synthesizing C1-C2 interlinked disaccharides has been developed in our lab, offering significant advancements in carbohydrate chemistry. We have demonstrated an efficient approach using readily available iodo-glycals and unsubstituted glycals as starting materials (Fig 2). The method involves reacting ester-protected donor molecules with ether-protected acceptors under a Pd-Ag catalytic system. This reaction yields C-disaccharides featuring a C-3 vinyl ether linkage. The process allows for subsequent ring opening through Lewis acid treatment, leading to the formation of π-extended conjugated orthogonally protected chiral ketones. Reduction and global deprotection leads to fully saturated disaccharides that are notably stable against acid hydrolysis, marking a significant achievement in the stability and utility of these complex molecules [Ref]. This new synthesis pathway not only simplifies the creation of intricate disaccharide structures but also enhances the stability and functionality of these compounds, paving the way for more advanced research and applications in carbohydrate chemistry.
References:
1. Rasool, B., Zargar, I. A., Hussain, N., & Mukherjee, D. (2023). Pd-catalyzed synthesis of hetero 1, 2-interlinked C-disaccharides by coupling of iodo glycals with glycals. Chemical Communications, 59(59), 9090-9093.
Month Year : August - 2024